Mars In The Loop : This Composite Of Images Spaced Some 5 To 9 Days Apart, From Late April (bottom Right)

Mars in the Loop : This composite of images spaced some 5 to 9 days apart, from late April (bottom right) through November 5 (top left), traces the retrograde motion of ruddy-colored Mars through planet Earth’s night sky. To connect the dots and dates in this 2018 Mars retrograde loop, just slide your cursor over the picture (and check out this animation). But Mars didn’t actually reverse the direction of its orbit. Instead, the apparent backwards motion with respect to the background stars is a reflection of the motion of the Earth itself. Retrograde motion can be seen each time Earth overtakes and laps planets orbiting farther from the Sun, the Earth moving more rapidly through its own relatively close-in orbit. On July 27, Mars was near its favorable 2018 parihelic opposition, when Mars was closest to the Sun in its orbit while also opposite the Sun in Earth’s sky. For that date, the frame used in this composite was taken during the total lunar eclipse. via NASA
More Posts from Science-is-magical and Others
ELI5:How come people can't be cryogenically frozen safely as the ice crystals destroy the cell membranes, but sex cells such as sperm are kept frozen for long periods of time yet remain functional?
I work in a lab where we freeze down cells all of the time. We freeze our cells in a medium that contains 5% DMSO, which among other things can be used as a cryoprotectant. However, DMSO is also toxic to cells at the concentrations necessary for cryoprotection. Consequently, when you freeze cells in DMSO, you add the DMSO medium at ice-cold temperatures and don’t allow the cells to warm up. When you later thaw the cells, you have to dilute out the DMSO as quickly as possible without causing osmotic shock, which can pop the cells. Such restrictions on freezing and thawing would basically be impossible to control at the level of a complete organism.
However, to contradict a lot of previous posts, individual cells can be recovered from freezing with high viability. When performed properly (and this varies quite a bit by cell type), you can expect >90% of cells to be alive following thaw.
The chemicals that allow cells to survive freezing are toxic to the body. Keeping the cells cold minimized the damage that this chemical does to the cells. With single cell solutions, adding the chemical at ice-cold temperatures and immediately diluting it out when you thaw the cells can keep 90% of the cells alive. There’s no way to do this with an intact body.
It’s also worth noting that this is probably not the only reason that this technique doesn’t scale to organisms.
Explain Like I`m Five: good questions, best answers.
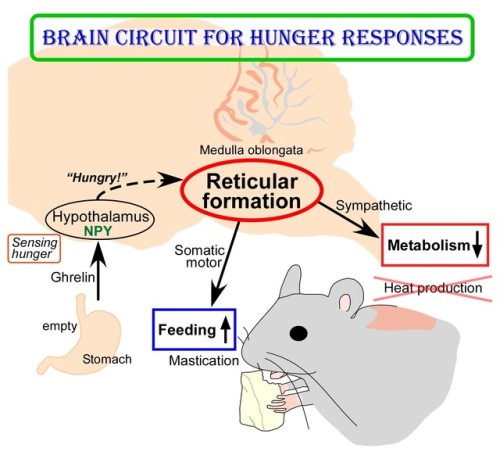
(Image caption: The empty stomach releases the hormone called ghrelin. By receiving ghrelin, the hypothalamus in the brain senses hunger and produces “hunger signaling” through the action of neuropeptide Y (NPY). The hunger signaling activates neurons in the reticular formation of the medulla oblongata, which then inhibit sympathetic output to reduce metabolic heat production and simultaneously provide masticatory motor rhythm to facilitate feeding. Credit: © 2017 Yoshiko Nakamura)
New Insights into Brain Circuit for Hunger Responses during Starvation
The human body responds to starving conditions, such as famine, to promote the chance of survival. It reduces energy expenditure by stopping heat production and promotes feeding behavior. These “hunger responses” are activated by the feeling of hunger in the stomach and are controlled by neuropeptide Y (NPY) signals released by neurons in the hypothalamus. However, how NPY signaling in the hypothalamus elicits the hunger responses has remained unknown.
Sympathetic motor neurons in the medulla oblongata are responsible for heat production by brown adipose tissue (BAT). Researchers centered at Nagoya University have now tested whether the heat-producing neurons respond to the same hypothalamic NPY signals that control hunger responses. They injected NPY into the hypothalamus of rats and tested the effect on heat production. Under normal conditions, blocking inhibitory GABAergic receptors or stimulating excitatory glutamatergic receptors in the sympathetic motor neurons induced heat production in BAT. After NPY injection, stimulating glutamatergic receptors did not produce heat, but inhibiting GABAergic receptors did. The study was reported in Cell Metabolism.
“This indicated that hypothalamic NPY signals prevent BAT thermogenesis by using inhibitory GABAergic inputs to sympathetic motor neurons,” study lead author Yoshiko Nakamura says.
Retrograde and anterograde tracing with fluorescent dyes revealed which brain region provided the inhibitory GABAergic inputs to heat-producing motor neurons.
“Tracing experiments showed that sympathetic motor neurons are directly innervated by GABAergic inputs from reticular nuclei in the medulla oblongata,” corresponding author Kazuhiro Nakamura explains, “selective activation of these GABAergic reticular neurons inhibits BAT thermogenesis.”
The researchers’ further findings showed that GABAergic inputs from medullary reticular neurons are involved in hypothalamic NPY-mediated inhibition of heat production in BAT. This hunger response circuit probably explains why anorexic individuals suffer from hypothermia.
Interestingly, stimulation of these medullary reticular neurons prompted rats to begin chewing and feeding. This effect was similar to injecting NPY into the hypothalamus, suggesting that hypothalamic NPY signaling activates reticular neurons in the medulla oblongata to promote feeding and mastication during the hunger response.
Abnormal activation of these neurons under non-starved conditions may contribute to obesity. Understanding these mechanisms could lead to development of more effective treatments for obesity.
Francium (new video) - Periodic Table of Videos
Interesting video on the element Francium and its discoverer Marguerite Perey.

It’s #InternationalKissingDay! Here’s some topical lipstick chemistry. More info/high-res image: http://wp.me/s4aPLT-lipstick



Women scientists made up 25% of the Pluto fly-by New Horizon team. Make sure you share this, because erasing women’s achievements in science and history is a tradition. Happens every day.
.
http://pluto.jhuapl.edu/News-Center/News-Article.php?page=20150712
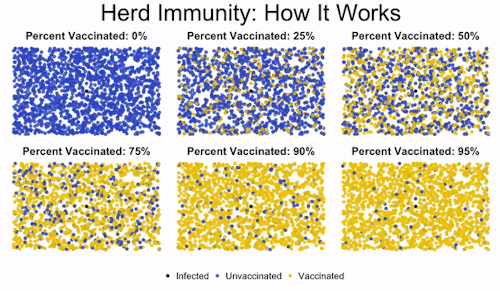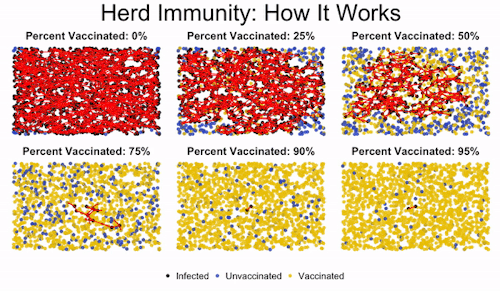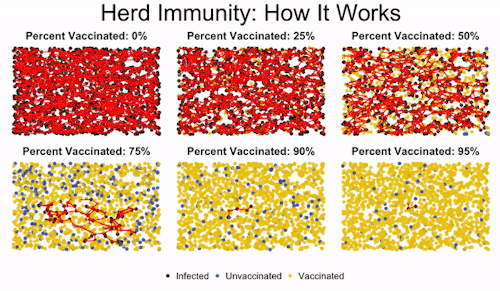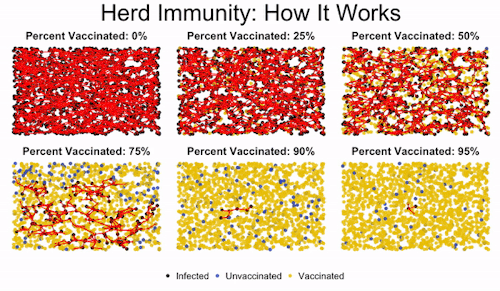
Herd immunity is the idea that if enough people get immunized against a disease, they’ll create protection for even those who aren’t vaccinated. This is important to protect those who can’t get vaccinated, like immunocompromised children.
You can see in the image how low levels of vaccination lead to everyone getting infected. Medium levels slow down the progression of the illness, but they don’t offer robust protection to the unvaccinated. But once you read a high enough level of vaccination, the disease gets effectively road-blocked. It can’t spread fast enough because it encounters too many vaccinated individuals, and so the majority of the population (even the unvaccinated people) are protected.
Find out more here.
Just Made a Bad Decision?
Most people experience anxiety in their lives. For some, it is just a bad, passing feeling, but, for many, anxiety rules their day-to-day lives, even to the point of taking over the decisions they make.
Scientists at the University of Pittsburgh have discovered a mechanism for how anxiety may disrupt decision making. In a study published in The Journal of Neuroscience, they report that anxiety disengages a region of the brain called the prefrontal cortex (PFC), which is critical for flexible decision making. By monitoring the activity of neurons in the PFC while anxious rats had to make decisions about how to get a reward, the scientists made two observations. First, anxiety leads to bad decisions when there are conflicting distractors present. Second, bad decisions under anxiety involve numbing of PFC neurons.
The data indicates that anxiety has an exquisitely selective effect on neuronal activity that supports decision making, says Bita Moghaddam, the lead author of the study and a professor in the Department of Neuroscience within the Kenneth P. Dietrich School of Arts and Sciences. Up to now, scientists have mostly studied anxiety in animal models in the context of fear and measured how brain cells react to a threatening situation. But human anxiety is devastating, not merely because of how the person feels, but also because it can interfere with nearly all aspects of daily life including decision making, Moghaddam says.
Pitt researchers studied this aspect of anxiety by monitoring the activity of a large number of neurons as rats made decisions about which choice was most optimal for receiving a reward. They compared behavior and neuronal activity in two groups: one group that had a placebo injection and another that got a low dose of an anxiety-inducing drug.
As with many people who suffer from anxiety but go through day-to-day life and make decisions, the anxious rats completed the decision-making task and, actually, did not do too badly. But they made far more mistakes when the correct choice involved ignoring distracting information. “A brain locus of vulnerability for these anxiety-induced mistakes was a group of cells in the PFC that specifically coded for choice. Anxiety weakened the coding power of these neurons.
“We have had a simplistic approach to studying and treating anxiety. We have equated it with fear and have mostly assumed that it over-engages entire brain circuits. But this study shows that anxiety disengages brain cells in a highly specialized manner.”
Perhaps, down the line, this better understanding of the brain mechanics behind anxiety and decision making, she says, could lead to better treatment of anxiety in people and, subsequently, better outcomes in the treatment of psychiatric disorders.
-
 r4ianec liked this · 1 year ago
r4ianec liked this · 1 year ago -
 coraniaid reblogged this · 3 years ago
coraniaid reblogged this · 3 years ago -
 deadlycarsmell reblogged this · 3 years ago
deadlycarsmell reblogged this · 3 years ago -
 lefthoundfriendbailiff liked this · 3 years ago
lefthoundfriendbailiff liked this · 3 years ago -
 science-is-magical reblogged this · 4 years ago
science-is-magical reblogged this · 4 years ago -
 w0manbeatur reblogged this · 4 years ago
w0manbeatur reblogged this · 4 years ago -
 blindedwithscience reblogged this · 4 years ago
blindedwithscience reblogged this · 4 years ago -
 blindedwithscience liked this · 4 years ago
blindedwithscience liked this · 4 years ago -
 anetteva reblogged this · 4 years ago
anetteva reblogged this · 4 years ago -
 serendipity1201 liked this · 4 years ago
serendipity1201 liked this · 4 years ago -
 25hs liked this · 4 years ago
25hs liked this · 4 years ago -
 macbethmoths reblogged this · 4 years ago
macbethmoths reblogged this · 4 years ago -
 macbethmoths liked this · 4 years ago
macbethmoths liked this · 4 years ago -
 totalmentedespreparada liked this · 4 years ago
totalmentedespreparada liked this · 4 years ago -
 amaramalakashur liked this · 4 years ago
amaramalakashur liked this · 4 years ago -
 lotusofthesun liked this · 4 years ago
lotusofthesun liked this · 4 years ago -
 xbluuetardisx liked this · 4 years ago
xbluuetardisx liked this · 4 years ago -
 meditationrelaxationmusic reblogged this · 4 years ago
meditationrelaxationmusic reblogged this · 4 years ago -
 horseshit-and-gunpowder reblogged this · 5 years ago
horseshit-and-gunpowder reblogged this · 5 years ago -
 horseshit-and-gunpowder liked this · 5 years ago
horseshit-and-gunpowder liked this · 5 years ago -
 cardamom5 liked this · 5 years ago
cardamom5 liked this · 5 years ago -
 pruebaslocass reblogged this · 5 years ago
pruebaslocass reblogged this · 5 years ago -
 joga-luce liked this · 5 years ago
joga-luce liked this · 5 years ago -
 lord-starquaad reblogged this · 5 years ago
lord-starquaad reblogged this · 5 years ago -
 lunarlilt reblogged this · 5 years ago
lunarlilt reblogged this · 5 years ago -
 fuckit-keepgoin reblogged this · 5 years ago
fuckit-keepgoin reblogged this · 5 years ago -
 slaughter-books liked this · 5 years ago
slaughter-books liked this · 5 years ago -
 flyawaylikethewind reblogged this · 5 years ago
flyawaylikethewind reblogged this · 5 years ago -
 joeby942 reblogged this · 5 years ago
joeby942 reblogged this · 5 years ago -
 joeby942 liked this · 5 years ago
joeby942 liked this · 5 years ago -
 magentalace420 reblogged this · 5 years ago
magentalace420 reblogged this · 5 years ago -
 chaillev liked this · 5 years ago
chaillev liked this · 5 years ago -
 periphery87 reblogged this · 5 years ago
periphery87 reblogged this · 5 years ago -
 4uimyt liked this · 5 years ago
4uimyt liked this · 5 years ago -
 theghostowl liked this · 5 years ago
theghostowl liked this · 5 years ago -
 rosebloodwater reblogged this · 5 years ago
rosebloodwater reblogged this · 5 years ago -
 atomic-moose reblogged this · 5 years ago
atomic-moose reblogged this · 5 years ago -
 intercal reblogged this · 5 years ago
intercal reblogged this · 5 years ago -
 dangara2610 liked this · 5 years ago
dangara2610 liked this · 5 years ago -
 nafsbluebery liked this · 5 years ago
nafsbluebery liked this · 5 years ago -
 kloudbby liked this · 5 years ago
kloudbby liked this · 5 years ago -
 metalzoic liked this · 5 years ago
metalzoic liked this · 5 years ago -
 gaytorade-official liked this · 5 years ago
gaytorade-official liked this · 5 years ago










