Chemical - Blog Posts
Chemical Space Gardens
You know that colorful crystal garden you grew as a kid?
Yeah, we do that in space now.
Chemical Gardens, a new investigation aboard the International Space Station takes a classic science experiment to space with the hope of improving our understanding of gravity’s impact on their structural formation.

Here on Earth, chemical gardens are most often used to teach students about things like chemical reactions.

Chemical gardens form when dissolvable metal salts are placed in an aqueous solution containing anions such as silicate, borate, phosphate, or carbonate.

Delivered to the space station aboard SpaceX’S CRS-15 cargo mission, the samples for this experiment will be processed by crew members and grown throughout Expedition 56 before returning to Earth.

Results from this investigation could provide a better understanding of cement science and improvements to biomaterial devices used for scaffolding, for use both in space and on Earth.
Follow the growth of the chemical garden and the hundreds of other investigations constantly orbiting above you by following @ISS_Research on Twitter.
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com
Ten Observations From Our Flying Telescope

SOFIA is a Boeing 747SP aircraft with a 100-inch telescope used to study the solar system and beyond by observing infrared light that can’t reach Earth’s surface.

What is infrared light? It’s light we cannot see with our eyes that is just beyond the red portion of visible light we see in a rainbow. It can be used to change your TV channels, which is how remote controls work, and it can tell us how hot things are.

Everything emits infrared radiation, even really cold objects like ice and newly forming stars! We use infrared light to study the life cycle of stars, the area around black holes, and to analyze the chemical fingerprints of complex molecules in space and in the atmospheres of other planets – including Pluto and Mars.

Above, is the highest-resolution image of the ring of dust and clouds around the back hole at the center of our Milky Way Galaxy. The bright Y-shaped feature is believed to be material falling from the ring into the black hole – which is located where the arms of the Y intersect.

The magnetic field in the galaxy M82 (pictured above) aligns with the dramatic flow of material driven by a burst of star formation. This is helping us learn how star formation shapes magnetic fields of an entire galaxy.

A nearby planetary system around the star Epsilon Eridani, the location of the fictional Babylon 5 space station, is similar to our own: it’s the closest known planetary system around a star like our sun and it also has an asteroid belt adjacent to the orbit of its largest, Jupiter-sized planet.

Observations of a supernova that exploded 10,000 years ago, that revealed it contains enough dust to make 7,000 Earth-sized planets!

Measurements of Pluto’s upper atmosphere, made just two weeks before our New Horizons spacecraft’s Pluto flyby. Combining these observations with those from the spacecraft are helping us understand the dwarf planet’s atmosphere.

A gluttonous star that has eaten the equivalent of 18 Jupiters in the last 80 years, which may change the theory of how stars and planets form.

Molecules like those in your burnt breakfast toast may offer clues to the building blocks of life. Scientists hypothesize that the growth of complex organic molecules like these is one of the steps leading to the emergence of life.

This map of carbon molecules in Orion’s Horsehead nebula (overlaid on an image of the nebula from the Palomar Sky Survey) is helping us understand how the earliest generations of stars formed. Our instruments on SOFIA use 14 detectors simultaneously, letting us make this map faster than ever before!
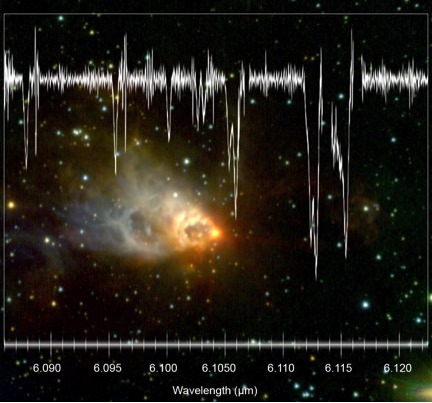
Pinpointing the location of water vapor in a newly forming star with groundbreaking precision. This is expanding our understanding of the distribution of water in the universe and its eventual incorporation into planets. The water vapor data from SOFIA is shown above laid over an image from the Gemini Observatory.

We captured the chemical fingerprints that revealed celestial clouds collapsing to form young stars like our sun. It’s very rare to directly observe this collapse in motion because it happens so quickly. One of the places where the collapse was observed is shown in this image from The Two Micron All Sky Survey.
Learn more by following SOFIA on Facebook, Twitter and Instagram.
Make sure to follow us on Tumblr for your regular dose of space: http://nasa.tumblr.com





100 Million X-Rays: NASA Co-Op #2 Week Three & Four
Jupiter orbital insertion, FIRST robotics coach visit and humidity sensor test prep have filled these past two weeks.
Drafting a fluid schematic complete with themocouples and pressure gauges I learn some fluid dynamics not expected to acquire as an electrical engineering and computer science major. The humidity sensor will be tested three ways - for 0% humidity with evaporating liquid nitrogen (Yah!), for ~ 50% with ambient room humidity down here in Houston, and >50% with ambient air being pulled through a water filled bubbler. Sensors will systemically be scattered to collect data and determine with a hefty amount of PV=NRTing if the humidity sensor works. After learning PV=NRT can only be used when you are certain the amount of water and vapor are equal to derive humidity we came up with the simple three part test matrix explained above.
My high school FIRST Robotics coach came to Johnson Space to tour some spacefaring robots, propulsion test center and space station mock ups (exact replicas of what is in space) at the Space Vehicle Mockup Facility! We also visited Houston’s Natural Science Museum and biked on Galveston.
Within a second of what was expected the Juno Spacecraft performed her tasks successfully and inserted into Jupiter’s orbit. This basketball court sized spacecraft will be exposed radiation equivalent to a human receiving 100 million X-Rays in a year. Juno also captured the first demonstration of celestial harmonic movement hypothesized by physics. Powered by solar energy this Juno is unique because most crafts that travel this far are radioisotope thermoelectrically powered. A critical part of this insertion was turning the solar arrays back toward the Sun post insertion.
WAYS TO GET INVOLVED
Watch…
Juno Media Briefing: https://youtu.be/I6uUEYOzipw
Juno Insertion: https://youtu.be/zfIqnpqPFbI
Juno Post Insertion Media Briefing: https://youtu.be/LH_uPWU5V3o
Apply for a NASA Internship: https://intern.nasa.gov/ossi/web/public/guest/searchOpps/
Apply for a NASA Co-Op (check back as it is updated as soon as one opens): http://nasajobs.nasa.gov/studentopps/employment/opportunities.htm
The two kinds of water

Credit: University of Basel
Pre-sorted ortho-water and para-water molecules with differently oriented nuclear spins (blue or red arrows) react with diazenylium ions (centre left) at different speeds.
–
Researchers from the University of Basel’s Department of Chemistry, Switzerland, has investigated how the two forms of water differ in terms of their chemical reactivity – the ability to undergo a chemical reaction. Both forms have almost identical physical properties, which makes their separation particularly challenging.
It is less well-known that water exists in two different forms (isomers) at the molecular level. The difference is in the relative orientation of the nuclear spins of the two hydrogen atoms. Depending on whether the spins are aligned in the same or opposite direction, one refers to ortho- or para-water.
The was made possible by a method based on electric fields. Using this, researchers were able to initiate controlled reactions between the pre-sorted water isomers and ultracold diazenylium ions (protonated nitrogen) held in a trap. During this process, a diazenylium ion transfers its proton to a water molecule. This reaction is also observed in the chemistry of interstellar space.
It was discovered that para-water reacts about 25% faster than ortho-water. This can be explained in terms of the nuclear spin also influencing the rotation of the water molecules. As a result, different attractive forces act between the reaction partners. Para-water is able to attract its reaction partner more strongly than the ortho-form, which leads to an increased chemical reactivity.